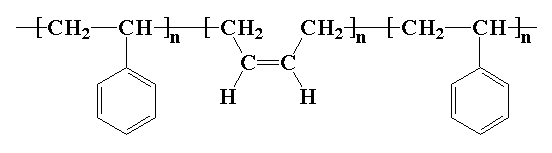
 Poly(styrene-butadiene-styrene), or SBS, is a hard rubber that"s used for things like the soles of shoes, tire treads, and other places where durability is important. It"s a type of copolymer called a block copolymer. Its backbone chain is made up of three segments. The first is a long chain of polystyrene, the middle is a long chain of polybutadiene, and the last segment is another long section of polystyrene. Here"s a picture:
Poly(styrene-butadiene-styrene), or SBS, is a hard rubber that"s used for things like the soles of shoes, tire treads, and other places where durability is important. It"s a type of copolymer called a block copolymer. Its backbone chain is made up of three segments. The first is a long chain of polystyrene, the middle is a long chain of polybutadiene, and the last segment is another long section of polystyrene. Here"s a picture:

Polystyrene is a tough hard plastic, and this gives SBS its durability. Polybutadiene is rubbery, and this gives SBS its rubber-like properties. In addition, the polystyrene chains tend to clump together. When one styrene group of one SBS molecule joins one clump, and the other polystyrene chain of the same SBS molecule joins another clump, the different clumps become tied together with rubbery polybutadiene chains. This gives the material the ability to retain its shape after being stretched.
SBS is made with some really clever chemistry called living anionic polymerization.
So you want to know how we make SBS rubber. Being the nice people we are, we"re going to tell you. It goes something like this: We start off using a technique called living anionic polymerization. A living polymerization is a polymerization that takes place without any termination reactions. This means that once all the monomer in your beaker is used up, and has been turned into polymer, the polymer chains are still active. This means that if you put more monomer in the beaker, it would add to the polymer and make the polymers bigger. This lets us do some tricks.
Wanna see what they are?
The first thing we have to do is make a chain of living polystyrene. This is done by polymerizing the monomer styrene with an anionic initiator like butyl lithium.
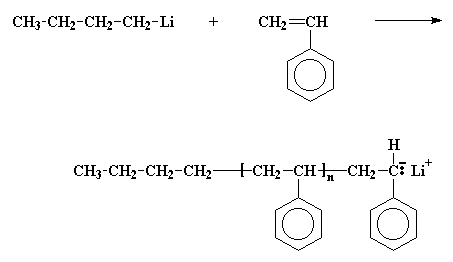
Remember now, this polystyrene chain is living, so if we add a second monomer to it, it"ll add to the polymer. So we"ll add some of the monomer butadiene.
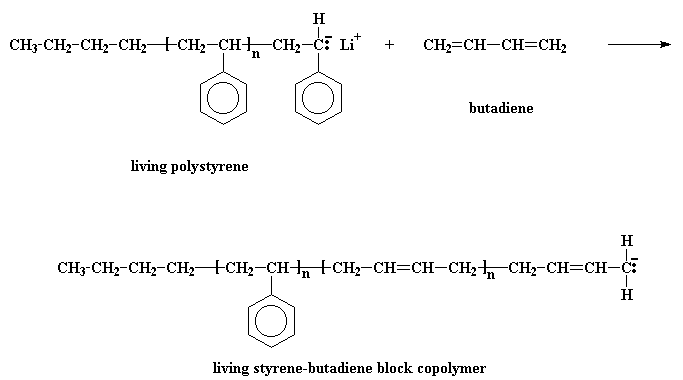
That gives us a living styrene-butadiene block copolymer.
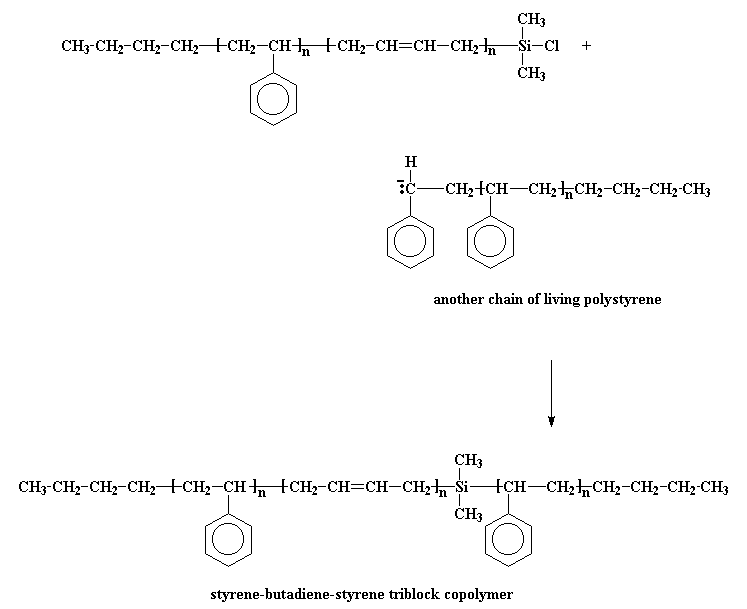
The next step is obvious: just add more styrene monomer, and get a styrene-butadiene-styrene triblock copolymer. Easy as pie. Funny thing, though. Although butadiene monomer will add to the anion at the end of a polystyrene chain, styrene monomer won"t add to the anion at the end of a living polybutadiene chain. This is most inconvenient. To get around this, we do a little trick: we"re going to react it with a compound called dichlorodimethylsilane.
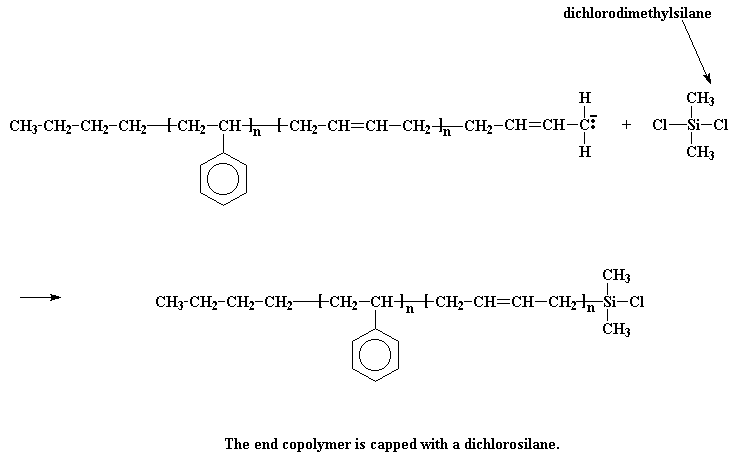
The anionic living chain kicks off a chlorine atom from the silane, and we get a chlorosilane end-capped polymer. So what good is that? Now our polymer is no longer living! It"s good because we can do something with this chlorosilane end-capped polymer. You see, if we take living polystyrene homopolymer, it will react with the chlorosilane end-capped polymer, just like the styrene-butadiene copolymer reacted with the dichlorodimethylsilane.
SBS is also a type of unusual material called a thermoplastic elastomer. These are materials that behave like elastomeric rubbers at room temperature, but when heated, can be processed like plastics. Most types of rubber are difficult to process because they are crosslinked. But SBS and other thermoplastic elastomers manage to be rubbery without being crosslinked, making them easy to process into nifty useful shapes.
Reference of this text is : http://pslc.ws/





